Quality process | Free your teams from the chaos of managing non-conformities









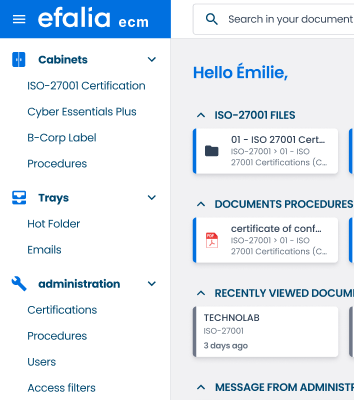
Control every noncompliance from detection to resolution
Start easily with a turnkey process (workflow + forms)

Easily change and connect your processes
Each organization has its own specificities: the Efalia Process Studio allows you to create, modify and evolve your processes for managing non-conformities without depending on IT.
You maintain control over your workflows and can connect them to your business ecosystem.
→ Intuitive configuration
Add or modify processing steps, change the order of validations, or create new circuits in a few clicks.
→ Integration with your tools
Automatically connect your processes to EDM to archive parts, trigger the sending of emails, or update your business databases.
→ Intelligent automation
Repetitive actions are automated, reducing processing times and the risk of errors.


Involve the right profiles and trace each action
The management of non-conformities involves several actors: declarants, managers, quality, production or logistics. Efalia Process allows you to define clear roles, involve the right people at the right time and maintain perfect traceability.
→ Differentiated user profiles
Each stakeholder can declare, complete, approve or validate according to their role, with appropriate visibility.
→ Delegation and automatic notifications
Tasks can be delegated, delays are reported, and automatic reminders prevent bottlenecks.
→ Traceability and complete history
Each action is time-stamped, allowing precise follow-up and easy preparation for quality audits.
Analytical dashboards
Visualize your key indicators in real time: resolution rate, average deadlines, distribution by type and service. Quickly identify trends and pain points. Turn non-conformities into opportunities for continuous improvement while ensuring compliance with your standards.
→ Full audit log
Each action is time-stamped and logged to meet ISO 9001/ISO 27001 requirements.
→ Automated reporting
Export your indicators for management reviews or external audits in a few clicks.
→ Structured corrective actions
Effectively plan, assign, and track all corrective actions until they are fully completed. Each action is documented with clearly identified deadlines and responsibilities.


















Examples of processes for non-conformities
Discover our complementary solutions
FAQS
Your data is hosted in France in SecNumCloud and HDS certified datacenters. We guarantee total GDPR and CNIL compliance, with isolation per customer and continuous supervision. All exchanges are encrypted and accesses are traced.
The intuitive interface allows immediate access to information via an efficient search and intelligent filters. Each user only accesses authorized data according to their profile, from any connected device.
Absolutely. Efalia Process connects natively to Efalia Doc to create a complete transversal EDM. You thus centralize all your quality documents (procedures, certificates, reports) while maintaining specialized workflows for non-conformities.
Yes, Efalia Process is designed API-first. We offer ready-to-use connectors for the main ERP and CMMS, as well as an open and documented API for your custom developments. The integration is seamless in your existing ecosystem.
Our digital solution goes beyond the simple recording of non-conformities by offering advanced analysis tools. You can easily identify recurring causes and weak points in your processes thanks to dynamic dashboards. Trends are visualized in real time, allowing targeted preventive actions.