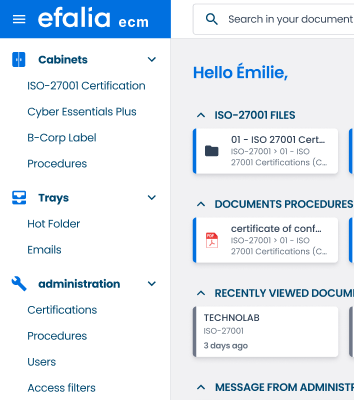
Quality safe, secure your critical documents








Store and protect your quality evidence in an NF 203 CFN certified safe
The sustainability and evidential value of your quality data
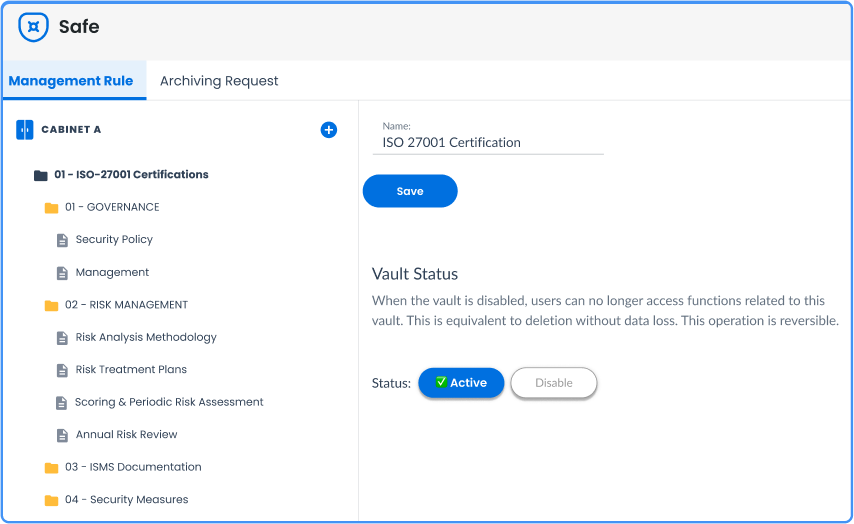
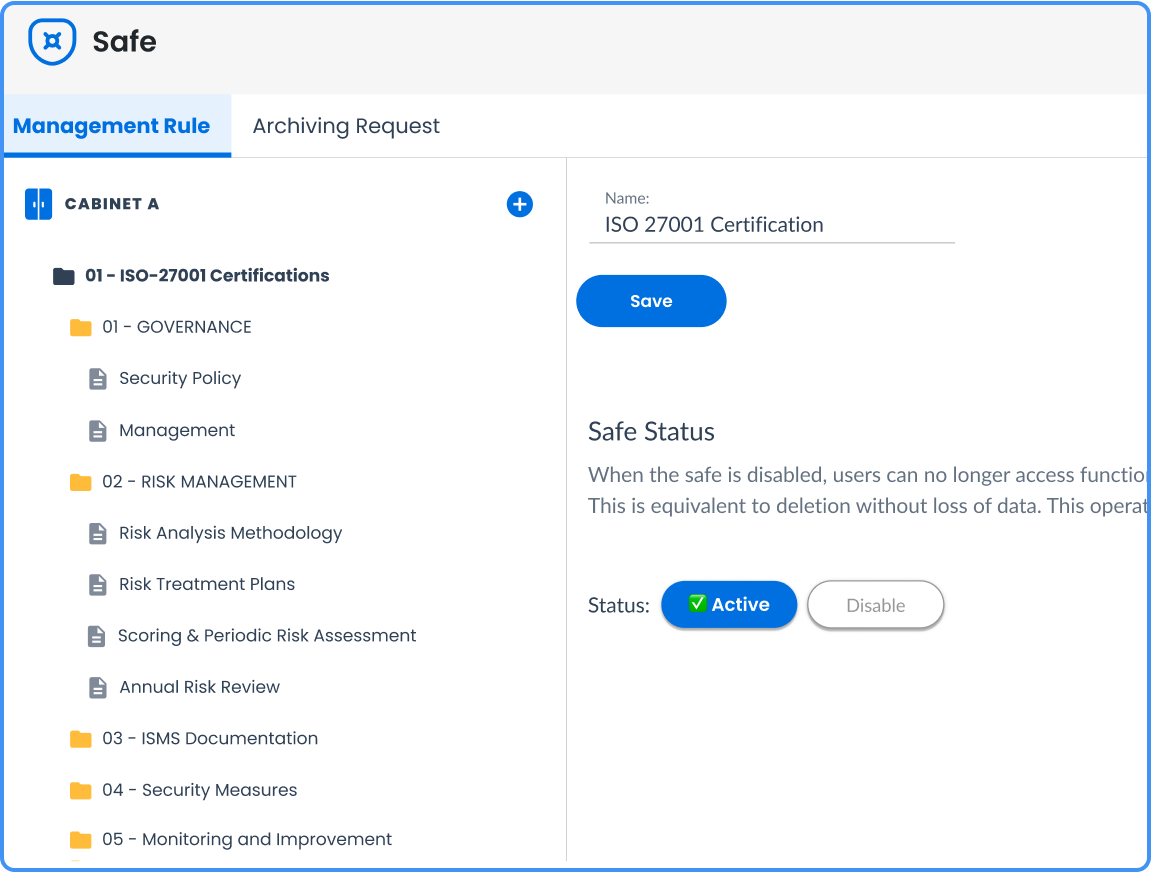
Secure and certified storage
Your quality documents are valuable and should be protected from loss, alteration, or unauthorized access.
→ Encryption and advanced security
Your files are protected in transit and at rest, with role-controlled access.
→ Versioning and historization
Each document maintains a complete history to prove its authenticity.
→ Controlled destruction
Manage the retention period and secure deletion according to your regulatory obligations.
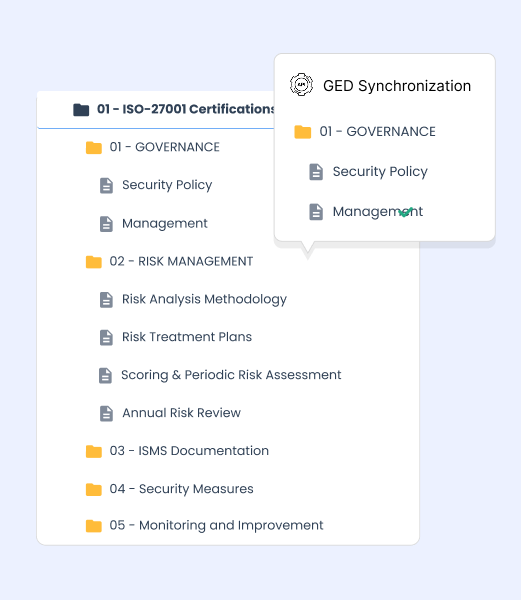
A safe integrated into your quality ecosystem
Optimize your quality processes thanks to a safe that communicates with your business tools.
Integration with EDM and SIRH
Automatic transfer of procedures, certificates or quality reports from your existing applications.
APIs and connectors
Possible connection with your ERP, homemade tools or industrial databases.
Notifications and automated transfer
When a critical document is validated, it is directly archived in the safe.
A key solution for ISO compliance
Prepare your quality audits with confidence with accessible and reliable evidence.
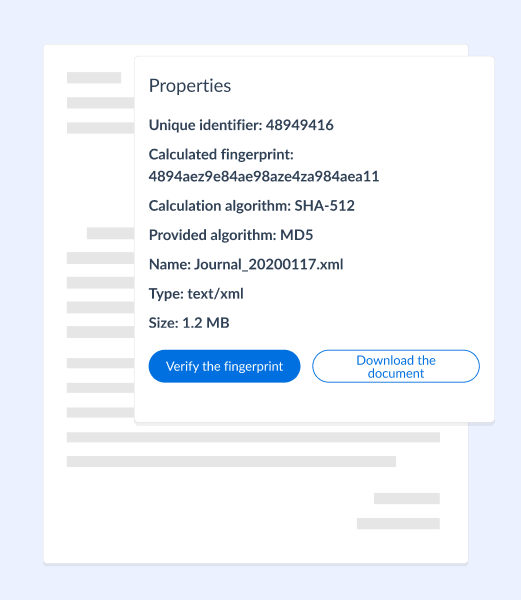
→ Immediate proofs of integrity
Each document is time-stamped, certified and searchable with its complete history.
→ ISO 9001/27001 compatibility
Keep your certificates of conformity, proofs of production or sensitive material sheets.
→ Speed during audits
An integrated search engine makes it possible to quickly provide the requested documents.


















Examples of use cases for quality safes
Discover our complementary solutions
FAQS
A quality digital safe like Efalia Safe guarantees the integrity and inviolability of documents thanks to a certified Reliable Audit Trail, offering probative value to the documents stored. Unlike an EDM, which mainly manages the documentary life cycle, our safe ensures the legal preservation of your critical quality documents, with electronic sealing mechanisms and an indisputable traceability required during certification audits.
Efalia Safe is fully customizable to adapt to your particular quality processes. You can configure document types, validation workflows, retention periods, and access levels according to the structure of your organization. Our modular approach allows you to create different “safe rooms” to separate your documents by quality area (product certificates, non-conformities, procedures, internal audits) while maintaining centralized governance. Our consultants support you in this configuration to ensure alignment with your business and regulatory requirements.
Absolutely. Efalia Safe has a powerful search engine that allows you to instantly find any document with its proofs of integrity. You can search by specific metadata (date, type of document, type of document, process, product concerned...), by full text or by personalized business criteria. Our system also makes it possible to prepare structured audit files in advance for a smooth presentation during controls.
Efalia Safe integrates natively with your existing systems thanks to its API-first architecture. We offer standard connectors for the main systems on the market (SAP, Microsoft, Oracle...) and can develop specific connectors for your business applications. This integration makes it possible to automate the archiving of your quality documents as soon as they are created in your source systems, without double entries and with complete traceability.
The documents stored in Efalia Safe benefit from optimal evidentiary value thanks to our certified Reliable Audit Trail and our electronic sealing mechanisms. The system guarantees the integrity, authenticity and traceability of each document, in accordance with the requirements of the Civil Code (art. 1366), the Commercial Code and the NF Z42-013 and ISO 14641 standards. In the event of a dispute, you can produce your documents with all the evidence of timestamp and non-alteration, significantly strengthening their legal admissibility.