ECM for Quality | Manage your quality documentation








What does quality document management software consist of?
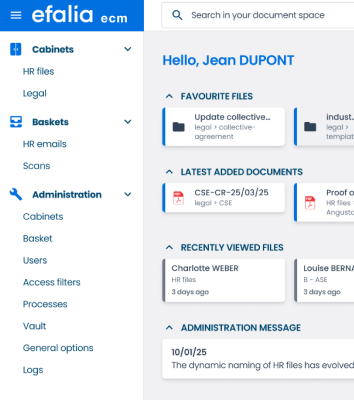
Your quality framework is always up to date and compliant thanks to your quality EDM
A Quality EDM designed for compliance and performance
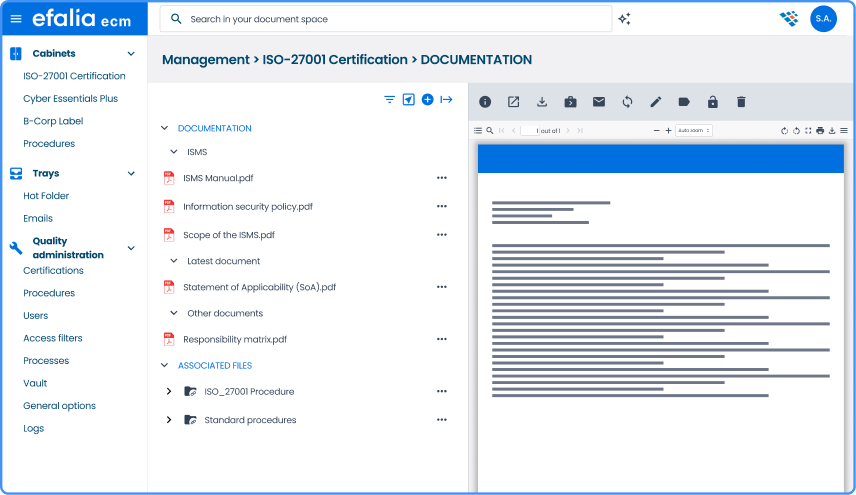
Structure and secure your quality reference system
All your quality documents (procedures, instructions, records) are organized in the tool via a single repository with a standards-compliant filing plan. No more duplicates or contradictory versions.
→ Smart and scalable ranking plan
Hierarchical organization of your documents according to your quality manual with automatic categorization. Intuitive navigation by process, type of document, or area of application.
→ Complete document lifecycle management
Automated status monitoring (draft, under review, validated, distributed, obsolete) with periodic review alerts. Retention of the complete history to meet traceability requirements.
→ Integration with your tools
Our software can be extended to other modules that can easily be integrated: supplier portal, BPM, digital safe, secure online signature and much more. The solution can also be extended to other external tools (office 365...).
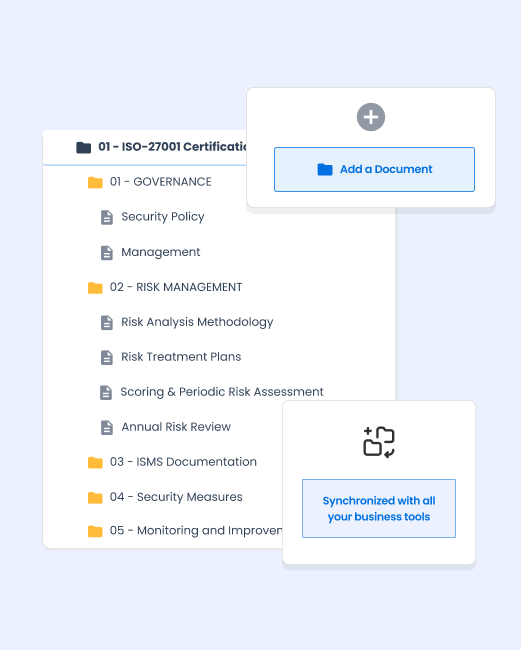
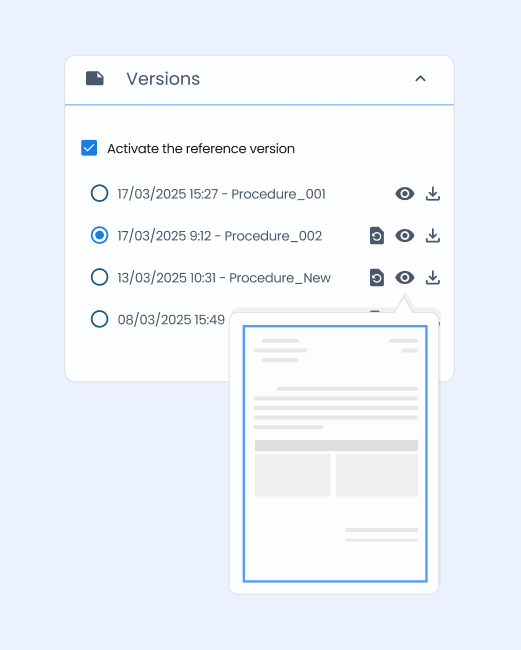
A reliable solution for your audits and certifications
Your Quality EDM becomes a real asset during regulatory controls and audits.
→ Full traceability
All actions are time-stamped and logged to meet ISO requirements.
→ Security and compliance
Encryption, fine rights management and sovereign hosting for your critical documents.
→ Comments and annotations system
Collaborative enrichment of documents with timestamp comments and attachments. Management of suggestions for improvement with follow-up of actions.
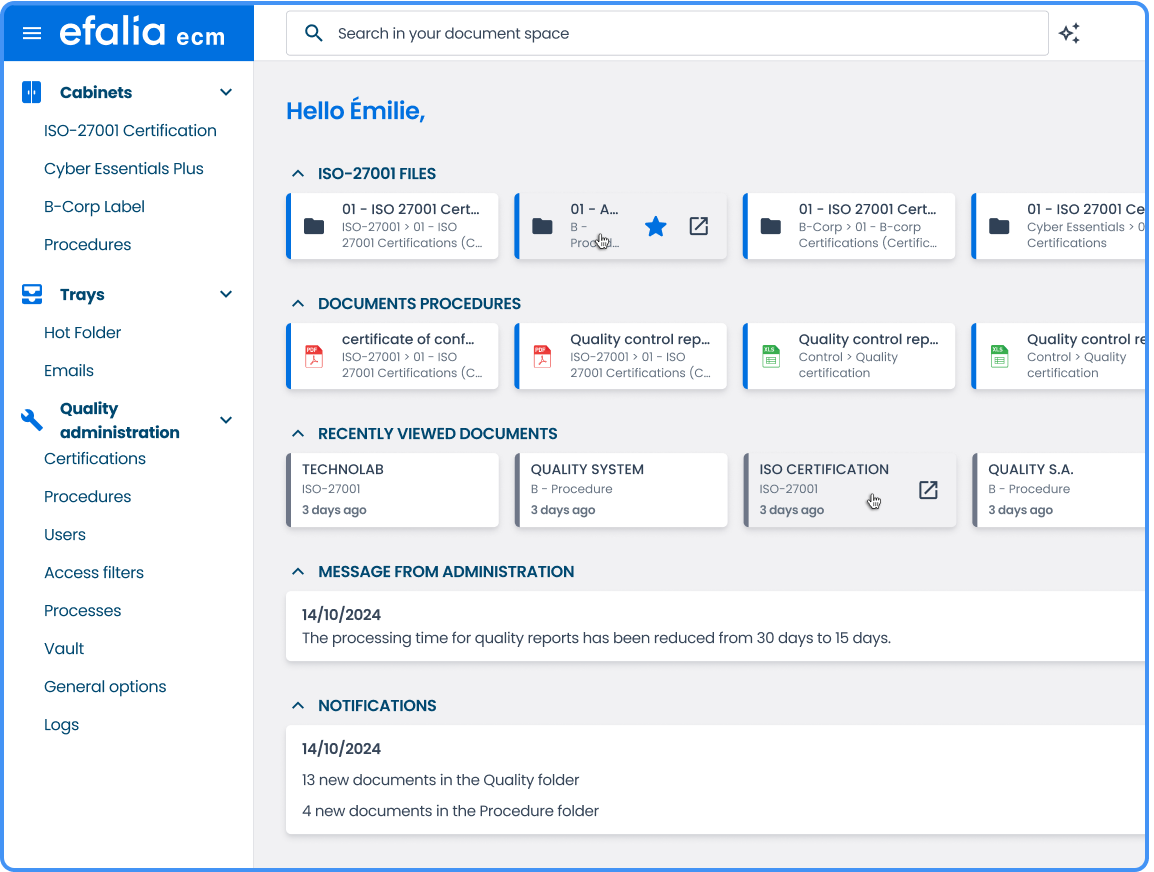
Controlled and accessible quality documentation
In a quality system, wasting time looking for a document or risking a bad version is critical.
With Efalia Quality EDM, your teams have instant and secure access to relevant information.
→ Advanced multi-criteria search
Find a document immediately by keywords, metadata, process, or validation status.
→ Fine management of user rights
Only the right person can access the right version, limiting the risks of uncontrolled distribution.
→ Controlled distribution
Only affected users receive the new versions, with a read receipt.
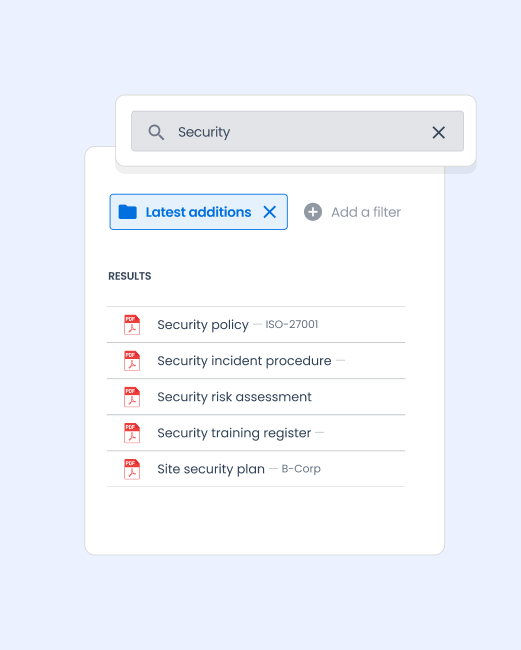

















Examples of use cases for quality EDM
Discover our complementary solutions
FAQS
Our quality electronic document management solution integrates advanced rights management based on roles and attributes, with data encryption at rest and in transit. The infrastructure is hosted in certified datacenters with daily encrypted backup and business continuity plan. All actions are recorded in an unalterable audit log.
Absolutely. Our responsive interface allows optimal access from tablets and smartphones. Simplified search and navigation by favorites facilitate access to critical documents even on the go. An offline mode allows the consultation of essential documents without an internet connection.
Yes, our modular architecture makes it easy to add other document domains (HR, Finance, Sales) in the same platform. You thus benefit from a unified documentary repository while maintaining the specificities of each business with their workflows and dedicated access rights.
Our EDM is natively designed with an API-first architecture offering more than 200 documented API points. This allows integration with your existing tools (ERP, HRIS, business software) and the development of third-party applications. Our native connectors cover the most common solutions on the market.
Yes, our software is specifically designed to help you meet the requirements of Chapter 7.5 of ISO 9001 concerning the management of documented information. It offers you all the tools you need to organize, control and trace your quality documents, thus facilitating your certification audits.