The management of non-conformities is essential for maintaining and improving the quality of a company's products and services. It makes it possible to detect, document, correct and prevent deviations from specified requirements, thus guaranteeing customer satisfaction. This article details a procedure for managing non-conformities, based on best practices as part of a quality approach, by addressing the objectives, scope of application, responsibilities and key stages of the process.
Objective of the NC procedure
The objective of this procedure is to define the steps for the detection, recording, curative and corrective treatment of non-conformities, as well as the measurement of the effectiveness of the actions taken. It aims to ensure the quality of products and services while meeting customer expectations.
Scope of application of the procedure
This procedure applies to all non-conformities detected in the company's processes, products, and services. It concerns all employees involved in these areas.
Definitions
Non-compliance (NC) : Deviation from specified requirements (standards, specifications, customer expectations).
Curative action : A measure taken to treat an NC immediately in order to contain it.
Corrective action : Action taken to eliminate the cause of a detected CN and to prevent its reoccurrence.
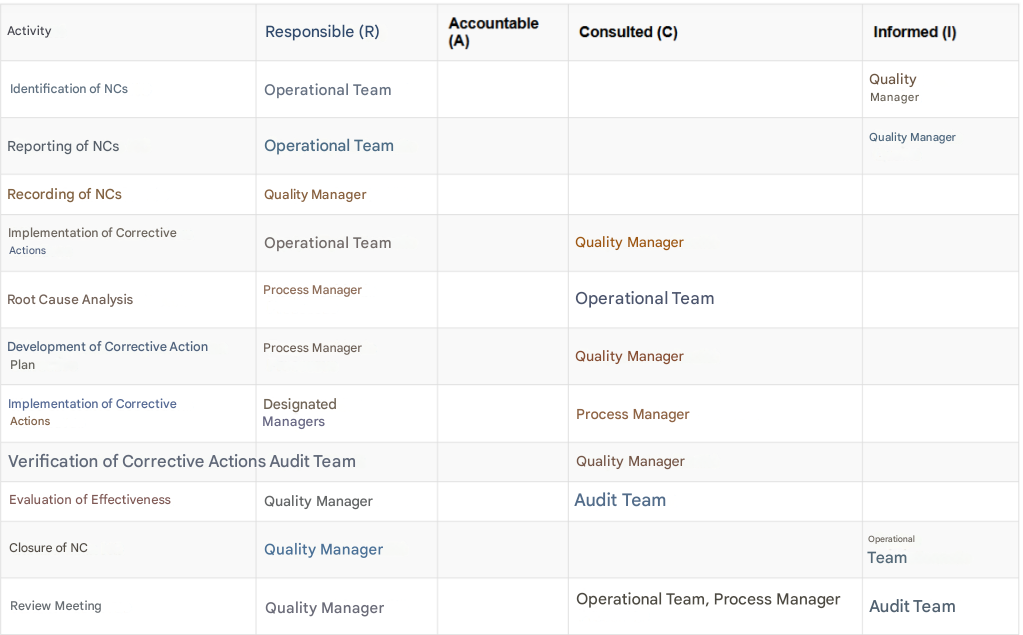
List of responsibilities
- Quality manager : Oversees the entire process of managing non-conformities.
- Operational team : Identifies and reports non-conformities.
- Process manager : Analyzes non-conformities and proposes corrective actions.
- Audit team : Verify the effectiveness of the corrective actions.
Above all, everyone's responsibilities must be clearly defined.
RACI matrix
Detailed procedure
✅ 1. Detection of Non-Conformities
- Identification : Anyone (operator, technician, auditor) identifying a non-compliance must report it immediately.
- Reporting : Use a non-compliance report form to document the non-compliance with relevant details (description, date, location, manager).
✅ 2. Registration of Non-Conformities
- Initial registration : The Quality Manager receives the NC form, verifies the information and records the non-compliance in the non-conformance register.
- Attribution of the reference : Each non-compliance receives a unique reference for follow-up.
✅ 3. Curative actions
- Immediate implementation : The operational team implements curative actions to contain non-compliance (isolation of the defective product, stopping the process, etc.).
- Curative action report : Document curative actions
✅ 4. Analysis and corrective actions
- Root cause analysis : The Process Manager performs root cause analysis using appropriate methods (5 Why, Ishikawa Diagram).
- Corrective action plan : Develop a corrective action plan including specific measures, managers and deadlines.
- Implementation : Corrective actions are implemented by designated officials.
✅ 5. Monitoring and measuring effectiveness
- Verification : The audit team carries out checks to ensure that the corrective actions have been properly implemented.
- Effectiveness Assessment : Measure the effectiveness of corrective actions through performance indicators (reduction in the rate of non-conformities, customer satisfaction, internal audits).
- Closing report : Document the results of the verification and evaluation and close the non-compliance in a register.
✅ 6. Review and continuous improvement
- Review meeting : Organize periodic meetings to analyze trends in non-conformities and identify opportunities for improvement.
- Process update : Adjust processes and procedures based on lessons learned and trends identified.
